What is Podiatric Medicine?
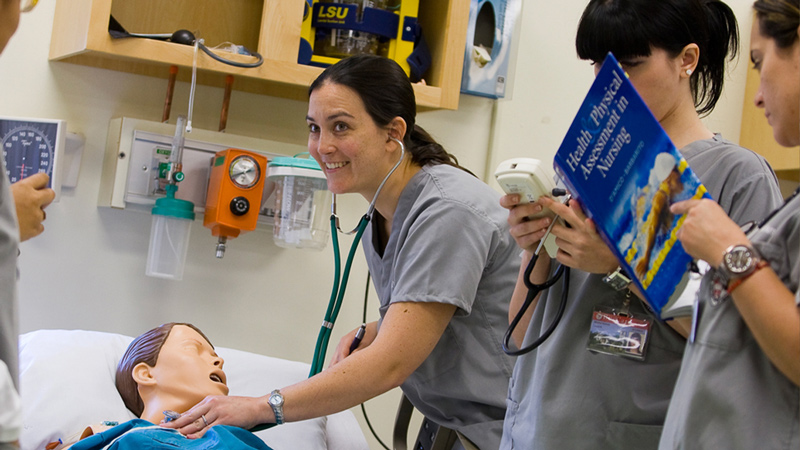
Podiatric medicine refers to the branch of medicine that is devoted to the diagnosis, treatment and care of disorders of the foot, ankle and related structures. A podiatrist is a doctor of podiatric medicine (DPM). Podiatrists are often the first to recognize arthritis, diabetes, and heart disease, skin disease, neurological ailments — all conditions that can affect the lower extremities. As a podiatrist, you can expect to be well trained to diagnose, treat, operate on, and prescribe medications for any disease, injury, deformity, or other condition of the foot or ankle. A career in podiatry offers the opportunity to specialize in many branches of podiatric medicine.
Specialties
-
Podiatric Orthopedics
Treatment of imperfect foot and leg structure and function using special footwear, orthotic and prosthetic devices, and physical therapy.
-

Podiatric Surgery
Utilization of modern operative procedures for the alleviation of various foot and ankle problems.
-

Podiatric Sports Medicine
The prevention, diagnosis, and treatment of lower extremity disorders in athletes. Barry University sponsors the only fellowship in podiatric sports medicine that is accredited by the Council on Podiatric Medical Education.
-

Podopediatrics
Prevention, diagnosis, and treatment of children's foot and leg problems.
-

Podiatric Primary Care
Prevention, diagnosis, and treatment of podiatric conditions as related to the total family health care environment.
-

Wound Care and Management
Care and prevention of wounds, ulcers, and injuries of the lower extremities, especially related to diabetes and other chronic systemic diseases.
-

Podiatric Research
Podiatric medical research seeks to develop and understand alternative and effective methods for diagnosis and treatment options to improve the health of patients under the care of podiatric physicians.